|
Magellan Diagnostics, Inc.
101 Billerica Ave., Bldg 4
N.
Billerica, MA 01862
Phone: 800-305-0197
Information request:
LeadCare@magellandx.com
|

www.MagellanDx.com
|
|
|
COMPANY DESCRIPTION: |
|
Magellan Diagnostics proudly delivers
a complete line of FDA-cleared blood lead testing systems. Our flagship
product, the CLIA-waived LeadCare® II system has
revolutionized pediatric lead testing, as real-time results and
preventive education are now a routine part of annual well-child visits.
Two new additions to the product line, LeadCare Ultra®
and LeadCare® Plus™, fulfill the needs of
clinical labs managing annual test volumes from a few hundred to 20,000.
Over 80% of labs currently performing blood lead testing use Magellan
Diagnostics’ systems.
|
|
|
Test
KIT REQUIREMENTS: |
|
Test kit name |
LeadCare II Blood Lead Test Kit |
 |
|
CLIA Classification |
CLIA Waived |
|
Analytes performed |
Lead |
|
CPT Code associated with each analyte |
83655 |
|
Test methodology |
Anodic Stripping Voltammetry (ASV) |
|
Sample volume |
50 µL |
|
Time to result |
3 minutes |
|
Units in which the test is reported |
µg/dL |
|
MSDS |
LeadCare II Lead Controls, LeadCare Treatment
Reagent
Available upon Request |
|
|
|
INSTRUMENT REQUIREMENTS: |
|
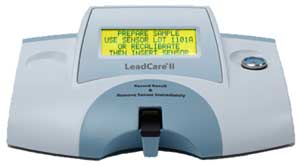
Instrument
name |
LeadCare II Blood Lead Analyzer |
 |
|
CLIA Classification |
CLIA Waived |
|
Size/Weight/Height |
9.0 in x 6.5 in x 3.5 in.; 2.5 lbs |
|
Specifications |
Reportable
range of 3.3-65 µg/dL;
AA batteries or 100-240 volts AC |
|
Data Transfer Capability |
N/A |
|
Features |
Electronic calibration, pre-packed test kits, portable,
results in 3 minutes |
|
Test Menu (Analytes
performed on instrument) |
Lead |
|
CPT Code for each Analyte |
83655 |
|
Test Methodology |
Electrochemical with disposable sensors |
|
Units in which
test results reported |
µg/dL |
|
|